Abstract
Introduction: Multiple myeloma (MM) is a plasma cell malignancy accounting for 10% of all hematological malignancies and characterized by significant inter and intra-patient heterogeneity. MM usually progresses from asymptomatic precursor stages, namely monoclonal gammopathy of undetermined significance (MGUS) and smoldering multiple myeloma (SMM). Previous recommendation by the IMWG indicated that bone marrow evaluation should be deferred in patients with low levels of IgG M-protein (< 1.5g/dL). Here, we sought to examine the clinical characteristics and progression patterns of patients who are defined as low risk MGUS by IMWG criteria but who also had a bone marrow biopsy performed.
Methods: A total of 244 patients with an IgG M-protein < 1.5 g/dL were identified from two cancer centers, Dana-Farber Cancer Institute, Boston, MA, USA and the Department of Clinical Therapeutics, Athens, Greece, in the period between 1998 to 2016. Patients who had undergone bone marrow biopsy immediately following the detection of M-protein in their serum were included in our cohort. Patients with IgA type or free light chain (FLC) disease were excluded because of their known high risk of progression. Continuous variables were summarized as median and interquartile range (IQR). Time-to-event endpoints were estimated using the method of Kaplan and Meier. The multivariate analysis was undertaken using Cox proportional-hazards regression, likelihood ratio model to identify independent predictors of progression among this group of patients. Subgroup analysis was performed according to the FLC ratio.
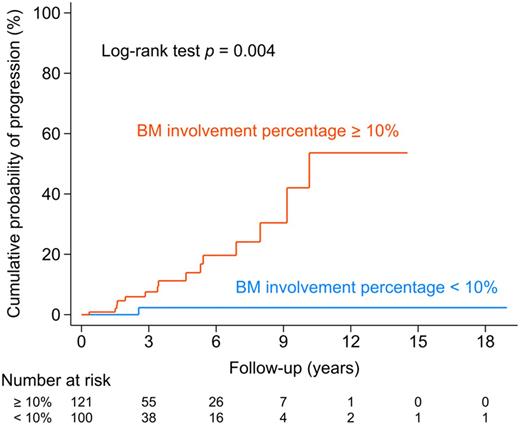
Results: Median age at diagnosis was 63 years and male to female ratio was 1.31. IgG k was present in 62.3%, while IgG l in 46.3% of patients. Median FLC ratio was 1.7 (IQR 0.9-3.2) and 51% of patients had an abnormal FLC ratio. In 54.5% of the patients, bone marrow infiltration by clonal plasma cells was ≥ 10%, reclassifying these patients as having SMM rather than MGUS. The time to progression to MM was significantly different between the patients with and without BM infiltration ≥ 10% in the Kaplan-Meier estimate (log-rank p= 0.004) (Figure 1). Subgroup analysis showed that patients with abnormal FLC ratio had a higher percentage of BM involvement (12% vs. 6%, p < 0.001) and 67% had ≥10% BM plasma cells vs. 44% of those with normal FLC ratio (p= 0.002); M-spike was also higher in those with abnormal FLC ratio (0.9 vs. 0.6, p < 0.001), as well as the probability of disease progression (median time-to-event: 9.2 years vs. not reached, log-rank p= 0.015).
Conclusion: Our findings indicate that over 50% of patients previously classified as low risk MGUS, based on the presence of an IgG monoclonal protein < 1.5 g/dL, would be reclassified as having SMM. Indeed, these patients had a higher risk of disease progression to symptomatic MM. Patients with less than 10% plasma cells and normal FLC had a negligible risk of progression. Thus, our data suggests that patients with M spike of < 1.5gm/dL should still undergo a bone marrow biopsy to complete their disease evaluation and help improve risk assessment for disease progression. Further validation of these results in other cohorts is warranted.
Kastritis: Prothena: Honoraria; Janssen: Honoraria; Genesis pharma: Honoraria; Takeda: Honoraria; Amgen: Honoraria, Research Funding. Laubach: Novartis, Takeda, Celgene, Onyx: Research Funding; Novartis, Takeda, Celgene: Consultancy. Anderson: Bristol-Myers Squibb: Membership on an entity's Board of Directors or advisory committees; MedImmune: Membership on an entity's Board of Directors or advisory committees; Gilead Sciences: Membership on an entity's Board of Directors or advisory committees; Oncopep: Other: scientific founder; Millenium Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; C4 Therapeutics: Other: scientific founder. Richardson: Takeda: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Oncopeptides AB: Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Dimopoulos: Amgen Inc, Celgene Corporation, Janssen Biotech Inc, Onyx Pharmaceuticals, an Amgen subsidiary, Takeda Oncology: Consultancy, Honoraria, Other: Advisory Committee: Amgen Inc, Celgene Corporation, Janssen Biotech Inc, Onyx Pharmaceuticals, an Amgen subsidiary, Takeda Oncology; Genesis Pharma: Research Funding; Novartis: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal